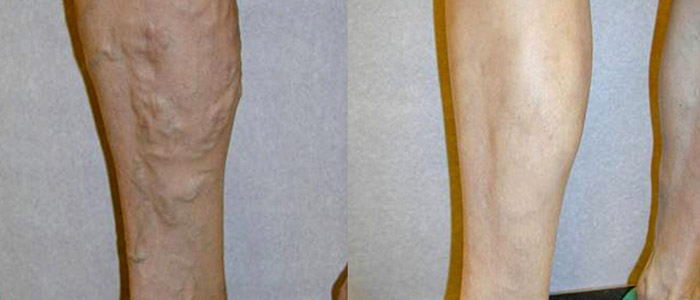
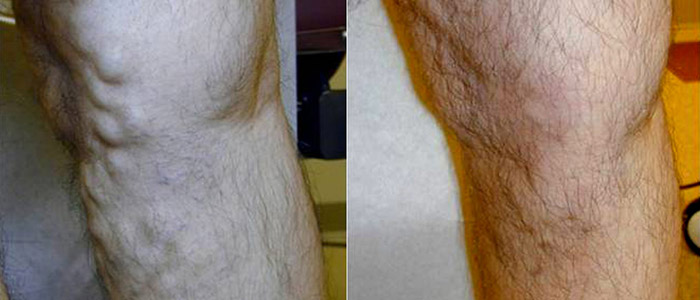
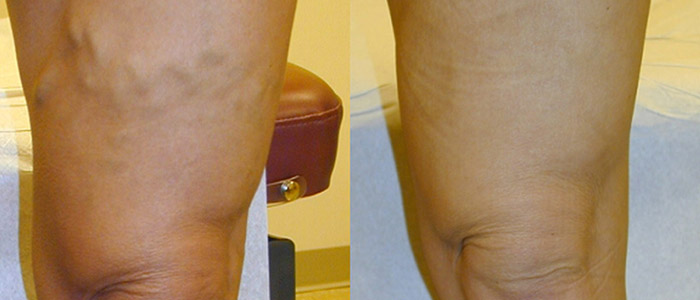
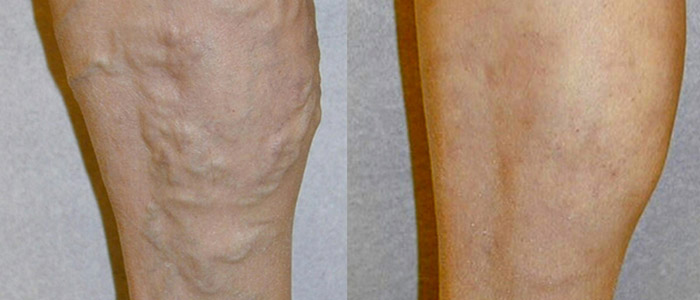
Where Will Vein Treatment Be by the Year 2015
There have been significant advances in modern medicine over the past few years, and the varicose vein treatment segment is no exception. While the current modalities of vein treatment are all effective and proven, there always remains room for improvement as scientists, engineers, and physicians learn more and have access to better technology. The most common modalities of vein treatment currently are thermal ablation, either by endovenous laser or radiofrequency, or injection of a sclerosant into the vein.
Typically sclerotherapy is performed for smaller veins, such as spider veins, while endovenous ablative therapies (VenaCure EVLT® laser vein treatment or VNUS Closure™ radiofrequency treatment) are used in larger veins. There are multiple factors that will play a role in determining where the next standard of treatment will fall. It is a combination of economics and of patient outcomes. Ideally, improved patient outcomes would drive improved economics, but this, unfortunately, is not always the case when it comes to Medicare or other insurance.
Additionally, medical device and pharmaceutical companies must have appropriate financial resources to continue to invest heavily in developing and testing new methods of treatment, with the goal of constantly improving care while reducing costs. Endovenous thermal ablative techniques have been proven for years to be very effective, with very low risk, and minimal pain or down time post-procedure. However, they do require the delivery of tumescent anesthesia. This adds overall time to the procedure and may cause slight discomfort to some patients. A few new methods of treating varicose veins include non-thermal techniques that will not require tumescent anesthesia. These new methods are innovative yet most are based on the same basic science of ablating the veins rather than restoring or repairing the valves.
The V-Block device is being tested in Europe with initial success. This device is implanted into the vein and adjunctive sclerotherapy is administered after it is inserted. It has a CE Mark approval, similar to FDA approval in the U.S., for use in the European Union. In its initial trial in humans after an average of 70 days of follow up, there were no DVTs reported and the great saphenous vein was successfully closed with no sign of venous reflux remaining. Another new treatment that has been generating some interest is essentially glue. It is called Cyanoacrylate Adhesive and has been used in other endovascular procedures in Europe for decades. The use of this adhesive in varicose vein treatment is new and still must undergo substantial testing to truly validate the long-term efficacy and safety.
However, initial results do show that it is promising as an alternative to thermal ablation and offers the cost reduction of not requiring major capital equipment such as a radiofrequency or laser generator. One device is a new mechanochemical delivery mechanism for delivering a sclerosing agent typically used in sclerotherapy of smaller veins or spider veins into the larger veins that cause tortuous varicosities. The device, called Clarivein, was used with Sotradecol® sclerosant in initial studies. Pain scores, as reported by the patients, during this trial were very low. Effectiveness after six months was good, but not yet as strong as existing ablative techniques.
While my colleagues and I continue to investigate new, improved methods of treating painful varicose veins and venous disease, we are still very pleased with the current modalities of thermal ablation with long-term, proven efficacy with minimal side effects reported. We are hopeful that Medicare will continue to recognize that these treatments are necessary to prevent debilitating and limb or even life threatening complications.