ClariVein® ablation

ClariVein® ablation is a revolutionary new procedure for treating varicose veins more quickly and simply than traditional procedures such as endovenous laser treatment (EVLT) or radiofrequency ablation. The latter treatments use high temperatures (120 degrees Celsius or more) to seal diseased veins, and thus require the use of multiple injections of tumescent anesthesia — a dilute Lidocaine mixture used to keep surrounding tissue from being damaged by the heat. These injections can be painful, and the heat treatments themselves can occasionally cause skin burns and nerve damage.
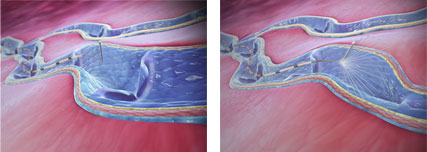
ClariVein treatment is a minimally invasive technique of a type called “mechano-chemical ablation.” During the procedure, the proprietary ClariVein® catheter is inserted into the affected vein and moved into place using ultrasound guidance. The tip of the catheter then rotates, causing the vein to go into spasm, after which a chemical is sprayed into the vein to seal it and prevent varicosity from recurring. This is similar to the way that sclerotherapy is used to treat smaller spider veins, but the action of the ClariVein catheter makes the process more effective by disrupting the lining of the vein and making it more receptive to the vein-closing drug.
The primary benefits of ClariVein® treatment are:
- Multiple anesthesia injections are no longer needed.
- No patient sensation of internal leg pressure due to anesthesia injections.
- 74% less post-operative pain and less bruising than with other treatments.
- Reduced procedure time.
- Zero risk of thermal injury, nerve, or skin damage.
- Results are visible almost immediately.
- You can return to your activities the same day.
Clinical trials have shown that success rates for varicose vein removal after two years are similar to those achieved by EVLT or radiofrequency ablation. The ClariVein(® device and procedure are FDA approved. To find out whether you may be a candidate for treatment using the ClariVein system, contact our offices and ask for an appointment with Dr. Kabnick.
